This is a description of the research at the Molecular Plant-Insect Interactions Laboratory at the University of Nebraska-Lincoln.
Our research focus is on identifying the key components/genes/signaling mechanisms that are involved in modulating plant defenses upon insect herbivory and to understand the mechanisms by which insect salivary proteins/effectors alter the plant defense responses.
Research
- Sorghum defense responses to phloem-feeding aphids. Sorghum, one of the world’s most important monocot crops grown for food, feed, and fuel, suffers severe yield losses due to attack by phloem-feeding insects, including aphids. This project will fill an important gap in current research by utilizing genomic resources to gain insight into the underlying genetic networks and phenotypic traits that contribute to sorghum resistance to aphids. To investigate this, we are utilizing the natural variation in a panel of sorghum inbred lines to elucidate the novel sources of sorghum resistance to sugarcane aphids (SCA). We are using a combination of molecular, biochemical, and electrophysiological approaches to enable better understanding of the genetic basis of sorghum resistance to aphids. The results from this project will provide improved insight as to how endogenous defenses and manipulation of defense signaling networks contribute to the development of more efficient and durable insect pest-resistant varieties of sorghum. This project is recently funded for more than $1.5 Million by the National Science Foundation Faculty Early Career Development Program (CAREER) Program.

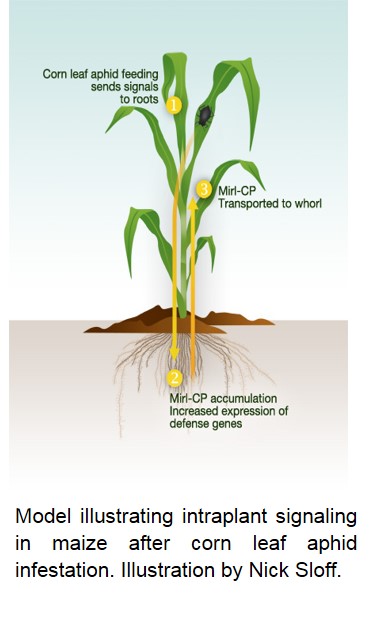
- Long-distance defense signaling in maize-insect interactions. We have identified that the aboveground feeding by aphids on maize rapidly sends a yet unidentified signal(s) to the roots that trigger belowground accumulation of the transcripts encoding insecticidal Maize Insect Resistance-Cysteine Protease (Mir1-CP), signifying a potential role of aboveground to belowground communication in maize defense against the phloem-feeding insects. Recently, we have also discovered that OPDA, an intermediate in the jasmonic acid biosynthesis pathway, can contribute to plant defense against insect pests by enhancing callose accumulation. Callose deposition is one of the defense mechanisms utilized by plants that contribute to sieve element occlusion and thus control infestation by phloem sap-feeding aphids. This study provided some interesting leads on how plant signaling mechanisms may limit insect performance by enhancing callose accumulation, thus providing an early line of defense against the insect.
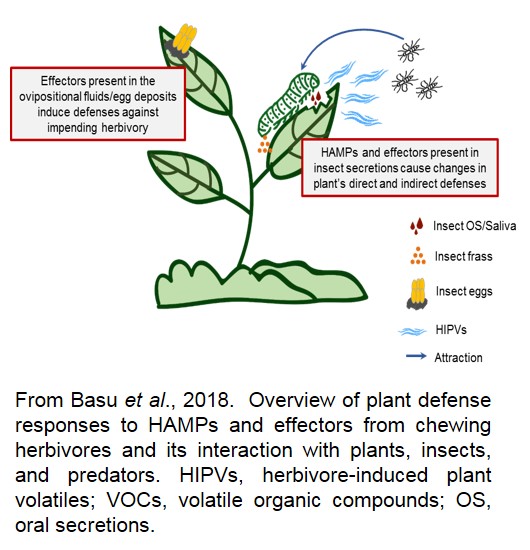
- Herbivore-Associated Molecular Patterns (HAMPs) in altering plant defenses. Plants have evolved complex defense mechanisms to overcome different stresses, including both biotic and abiotic stresses. At the same time, insects produce a suite of Herbivore-Associated Molecular Patterns (HAMPs) present in the insect oral secretions (regurgitant), saliva, and/or frass may either amplify/suppress the induced plant defenses. We are currently employing proteomic techniques to identify the protein components of caterpillar saliva and frass that modulate plant defenses in sorghum.
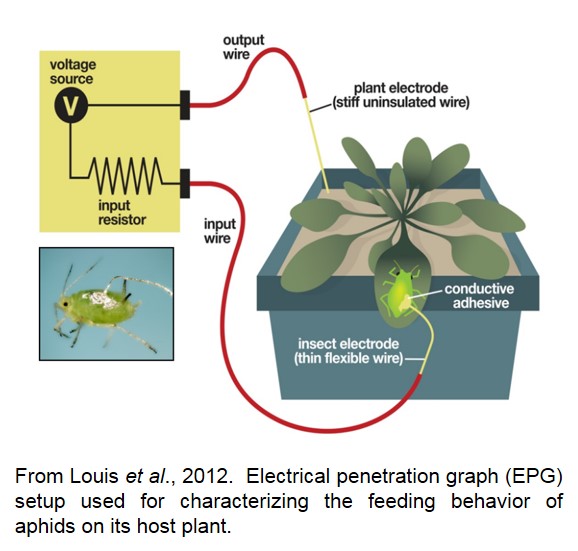
- Electrical Penetration Graph (EPG) as a tool to study plant-insect interactions. EPG technique is a potent technique to investigate the specifics of plant resistance to piercing/sucking insects. Monitoring this probing behavior is critical in understanding the localization of plant defenses and to determine how plants engage their defense components to restrict aphid feeding. Louis lab has a direct current (DC)-EPG system, which measures the electrical resistance fluctuations produced by the penetrating insect as well as the electromotive force (EMF) signal components that are generated as a result of the aphid feeding. This responsiveness to EMF components is utilized to differentiate between intracellular and intercellular stylet tip positions. When the aphid stylet is inserted intercellularly, the voltage is positive and when inserted intracellularly, the voltage is negative, resulting in potential drops in the signal which is correlated with the physiological condition and defense status of the host.